Recently, the team of Professor Qi Qingsheng from the State Key Laboratory of Microbial Technology at Shandong University published their research findings titled "Improving the Substrate Binding Pocket Flexibility of Leaf-Branch Compost Cutinase for Efficient and Complete Degradation of Commercial PBAT Plastic" online in the international journal Chemical Engineering Journal. This work provides a novel, highly active catalyst for the biodegradation and recycling of PBAT plastic waste. This research was jointly supervised by Professor Qi Qingsheng and Associate Researcher Su Tianyuan from the State Key Laboratory of Microbial Technology at Shandong University. PhD student Han Yuanfei from the Key Laboratory is the first author of the paper. Shandong University is the primary completion unit and the corresponding author unit for this study.
Poly(butylene adipate-co-terephthalate) (PBAT), as a biodegradable plastic, is a mainstream substitute for traditional non-biodegradable plastics, particularly for replacing polyethylene and polyvinyl chloride agricultural plastic films. PBAT plastic possesses excellent ductility and thermal stability and has been widely used in the production of agricultural films. Its significant advantage of being biodegradable is of great importance for sustainable agricultural development. However, incomplete degradation of PBAT in natural environments can easily form microplastics, and its degradation intermediate, terephthalic acid (TPA), has certain biotoxicity. Accumulation of TPA in the environment poses a potential threat to ecosystems. More importantly, the raw materials for synthesizing PBAT mainly come from non-renewable petrochemical resources. Discarding large amounts into the environment would severely exacerbate carbon emissions. To address these challenges, researchers both domestically and internationally have begun actively studying and designing efficient PBAT-degrading enzymes.
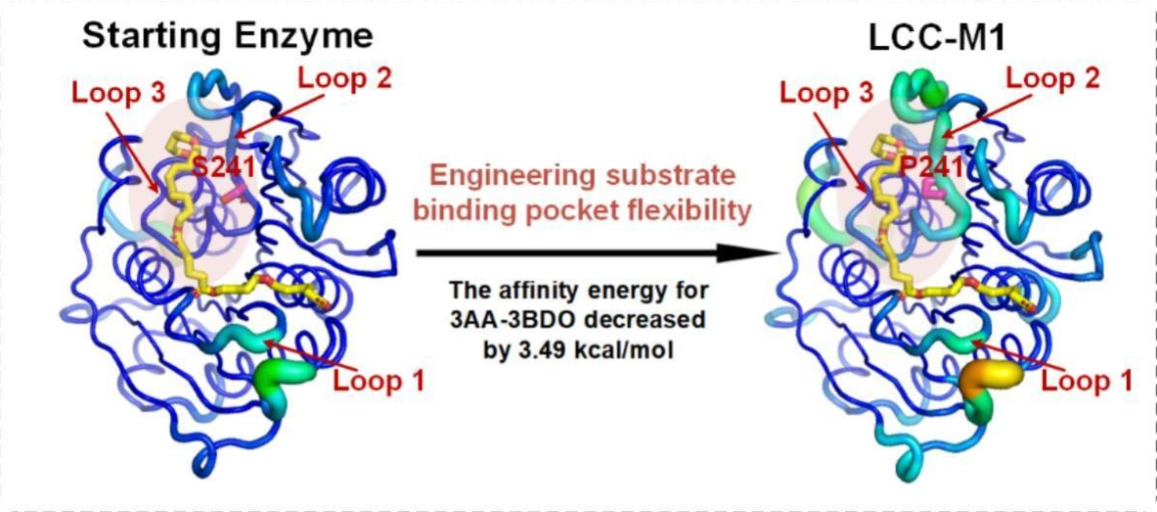
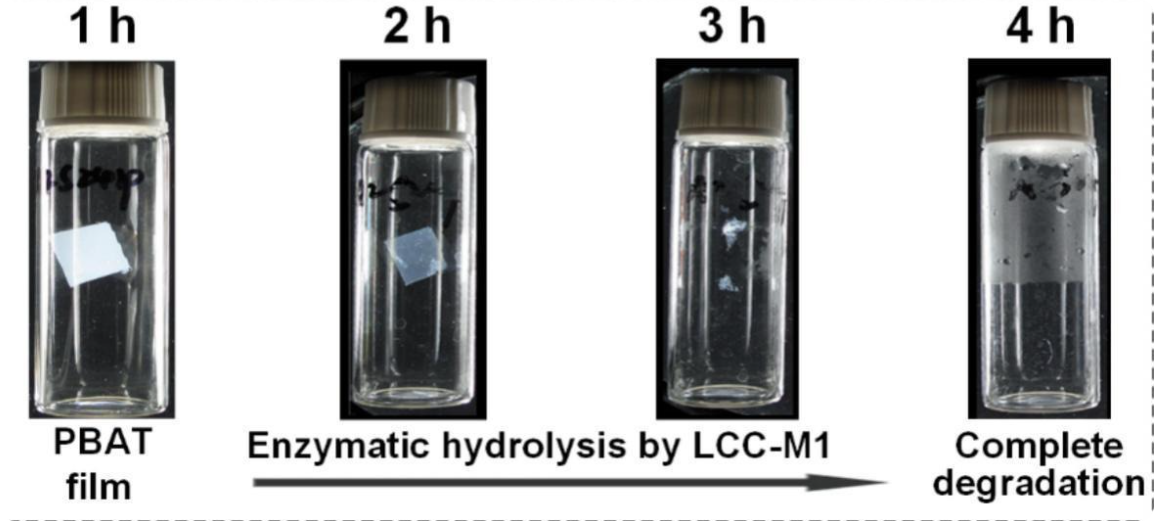
Substrate Binding Pocket Engineering Enhances PBAT Degradation Activity of Plastic-Degrading Enzymes
The PBAT molecule contains both aliphatic and aromatic ester bond structures. Therefore, developing a plastic hydrolase capable of simultaneously and efficiently degrading these two types of ester bonds is key to the efficient recycling of PBAT plastic waste. Previously, this team developed an efficient PET-degrading enzyme, LCC-A2 (ACS Catalysis, 2024), which can degrade over 90% of post-consumer PET plastic waste within 3.3 hours. In this study, by enhancing the flexibility of the key loop region in the substrate binding pocket of LCC-A2 to improve its adaptability for the PBAT substrate, the mutant LCC-M1 was obtained. This mutant exhibits outstanding degradation capability for both types of ester bonds in PBAT. It can completely degrade commercial PBAT film without any pretreatment within 4 hours, and fully degrade commercial PBAT/PLA blended plastic bags within 3 hours. Over 99% of the degradation products are final degradation products. LCC-M1 is currently the most efficient PBAT-degrading enzyme reported. The plastic-degrading enzyme substrate binding pocket modification strategy developed in this study and the obtained PBAT-degrading enzyme mutant are of significant importance for addressing the increasingly severe problem of plastic pollution.
This research work was supported by grants from the National Key Research and Development Program of China, the National Natural Science Foundation of China, the Shandong Provincial Taishan Scholar Young Expert Project, the SKLMT Frontier and Challenge Project, and the Shandong Provincial Natural Science Foundation, among others.


